– 15 –
STAINLESS STEEL EQUIPMENT CARE AND CLEANING
(Supplied courtesy of NAFEM. For more information, visit their web site at www.nafem.org)
Contrary to popular belief, stainless steels ARE susceptible to
rusting.
Corrosion on metals is everywhere. It is recognized quickly on iron
and steel as unsightly yellow/orange rust. Such metals are called
“active” because they actively corrode in a natural environment when
their atoms combine with oxygen to form rust.
Stainless steels are passive metals because they contain other
metals, like chromium, nickel and manganese that stabilize the
atoms. 400 series stainless steels are called ferritic, contain
chromium, and are magnetic; 300 series stainless steels are called
austenitic, contain chromium and nickel; and 200 series stainless,
also austenitic, contains manganese, nitrogen and carbon. Austenitic
types of stainless are not magnetic, and generally provide greater
resistance to corrosion than ferritic types.
With 12-30 percent chromium, an invisible passive fi lm covers the
steel’s surface acting as a shield against corrosion. As long as the
fi lm is intact and not broken or contaminated, the metal is passive
and stain-less. If the passive fi lm of stainless steel has been broken,
equipment starts to corrode. At its end, it rusts.
Enemies of Stainless Steel
There are three basic things which can break down stainless steel’s
passivity layer and allow corrosion to occur.
1. Mechanical abrasion
2. Deposits and water
3. Chlorides
Mechanical abrasion means those things that will scratch a steel
surface. Steel pads, wire brushes and scrapers are prime examples.
Water comes out of the faucet in varying degrees of hardness.
Depending on what part of the country you live in, you may have hard
or soft water. Hard water may leave spots, and when heated leave
deposits behind that if left to sit, will break down the passive layer and
rust stainless steel. Other deposits from food preparation and service
must be properly removed.
Chlorides are found nearly everywhere. They are in water, food
and table salt. One of the worst chloride perpetrators can come from
household and industrial cleaners.
So what does all this mean? Don’t Despair!
Here are a few steps that can help prevent stainless steel rust.
1. Use the proper tools.
When cleaning stainless steel products, use non-abrasive tools.
Soft cloths and plastic scouring pads will not harm steel’s passive
layer. Stainless steel pads also can be used but the scrubbing
motion must be in the direction of the manufacturers’ polishing
marks.
2. Clean with the polish lines.
Some stainless steel comes with visible polishing lines or “grain.”
When visible lines are present, always scrub in a motion parallel
to the lines. When the grain cannot be seen, play it safe and use
a soft cloth or plastic scouring pad.
3. Use alkaline, alkaline chlorinated or non-chloride
containing cleaners.
While many traditional cleaners are loaded with chlorides, the
industry is providing an ever-increasing choice of non-chloride
cleaners. If you are not sure of chloride content in the cleaner
used, contact your cleaner supplier. If your present cleaner
contains chlorides, ask your supplier if they have an alternative.
Avoid cleaners containing quaternary salts; it also can attack
stainless steel and cause pitting and rusting.
4. Treat your water.
Though this is not always practical, softening hard water can do
much to reduce deposits. There are certain fi lters that can be
installed to remove distasteful and corrosive elements. To insure
proper water treatment, call a treatment specialist.
5. Keep your food equipment clean.
Use alkaline, alkaline chlorinated or non-chloride cleaners
at recommended strength. Clean frequently to avoid build-
up of hard, stubborn stains. If you boil water in stainless steel
equipment, remember the single most likely cause of damage
is chlorides in the water. Heating cleaners that contain chlorides
have a similar effect.
6. Rinse, rinse, rinse.
If chlorinated cleaners are used, rinse and wipe equipment and
supplies dry immediately. The sooner you wipe off standing water,
especially when it contains cleaning agents, the better. After
wiping equipment down, allow it to air dry; oxygen helps maintain
the stainless steel’s passivity fi lm.
7. Never use hydrochloric acid (muriatic acid) on stainless
steel.
8. Regularly restore/passivate stainless steel.
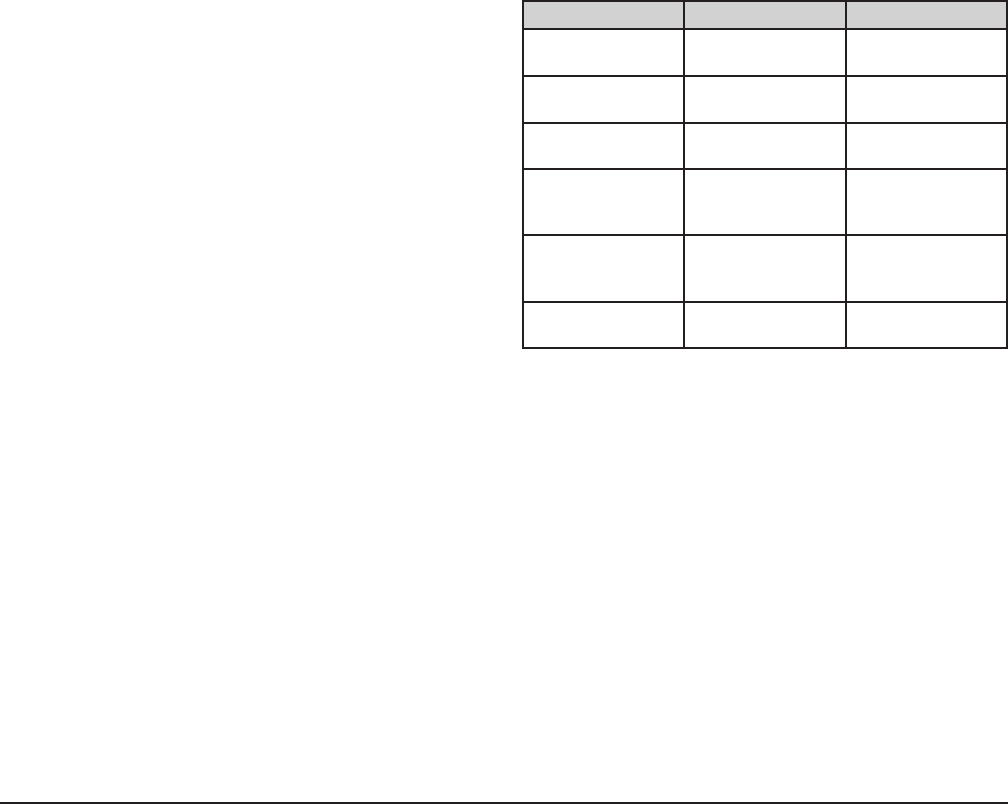
Job Cleaning Agent Comments
Routine cleaning Soap, ammonia,
detergent, Medallion
Apply with soft
cloth or sponge.
Fingerprints
and smears
Arcal 20, Lac-O-
Nu Ecoshine
Provides barrier fi lm
Stubborn stains
and discoloration
Cameo, Talc, Zud,
First Impression
Rub in direction
of polish lines.
Grease and fatty
acids, blood,
burnt-on foods
Easy-off, DeGrease
It Oven Aid
Excellent removal
on all fi nishes
Grease and Oil Any good
commercial
detergent
Apply with soft
cloth or sponge.
Restoration/
Passivation
Benefi t, Super
Sheen
Review
1. Stainless steels rust when passivity (fi lm-shield) breaks down
as a result of scrapes, scratches, deposits and chlorides.
2. Stainless steel rust starts with pits and cracks.
3. Use the proper tools. Do not use steel pads, wire brushes or
scrapers to clean stainless steel.
4. Use non-chlorinated cleaners at recommended concentrations.
Use only chloride free cleaners.
5. Soften your water. Use fi lters and softeners whenever
possible.
6. Wipe off cleaning agent(s) and standing water as soon as
possible. Prolonged contact causes eventual problems.
To learn more about chloride-stress corrosion and how to prevent it,
contact the equipment manufacturer or cleaning materials supplier.
Developed by Packer Engineering, Naperville, Ill., an independent
testing laboratory.


















